
Originally Published Via Mondaq
Proposals have been lodged with the Jersey legislature to amend the Proceeds of Crime (Jersey) Law 1999 expressly excluding lawful cannabis related activity from the definition of “criminal conduct”. This article is an in-depth review of the cannabis industry and Jersey’s relationship to it.
 The MDAC concluded that it would be reasonable and proportionate to permit small amounts of THC and other cannabinoids to be present in CBD products sold as food supplements. The 2019 Order amended the 2009 Order to permit the preparation of cannabidiol which (i) has its ingredients clearly labelled, (ii) contains not more than 3% cannabinol and/or cannabinol derivatives relative to its cannabidiol content, by weight, (iii) does not contain any other controlled drug, (iv) does not contain any plant material visible to the naked eye, and (v) has the matter of the combined weight attested by an official certificate of analysis, or by the Official Analyst appointed under Article 2 of the Food Safety (Jersey) Law 1966.
Any CBD preparation meeting the above requirements should be exempted from controls on importation, possession, supply, administration. However, exportation should still be unlawful except when in accordance with a licence issued under the provisions of 2009 Order.
INVESTMENT BY JERSEY VEHICLES INTO CANNABIS AND OTHER CANNABIS-RELATED ASSETS OVERSEAS AND THE PROVISION OF RELATED FINANCIAL SERVICES
It has been recognised that the Proceeds of Crime (Jersey) Law 1999 (POCL) has the potential to create dual criminality for persons who receive income in Jersey from activities which occur legally abroad but which are unlawful in Jersey.
Jersey investors receiving income or dividends from investments in overseas medicinal cannabis companies will also need to ensure that they do not fall foul of POCL.
There are a number of offences under POCL, including:
The MDAC concluded that it would be reasonable and proportionate to permit small amounts of THC and other cannabinoids to be present in CBD products sold as food supplements. The 2019 Order amended the 2009 Order to permit the preparation of cannabidiol which (i) has its ingredients clearly labelled, (ii) contains not more than 3% cannabinol and/or cannabinol derivatives relative to its cannabidiol content, by weight, (iii) does not contain any other controlled drug, (iv) does not contain any plant material visible to the naked eye, and (v) has the matter of the combined weight attested by an official certificate of analysis, or by the Official Analyst appointed under Article 2 of the Food Safety (Jersey) Law 1966.
Any CBD preparation meeting the above requirements should be exempted from controls on importation, possession, supply, administration. However, exportation should still be unlawful except when in accordance with a licence issued under the provisions of 2009 Order.
INVESTMENT BY JERSEY VEHICLES INTO CANNABIS AND OTHER CANNABIS-RELATED ASSETS OVERSEAS AND THE PROVISION OF RELATED FINANCIAL SERVICES
It has been recognised that the Proceeds of Crime (Jersey) Law 1999 (POCL) has the potential to create dual criminality for persons who receive income in Jersey from activities which occur legally abroad but which are unlawful in Jersey.
Jersey investors receiving income or dividends from investments in overseas medicinal cannabis companies will also need to ensure that they do not fall foul of POCL.
There are a number of offences under POCL, including:
INTERNATIONAL CANNABIS POLICY
The cultivation and production of cannabis for recreational purposes is now lawful in a number of countries including Canada, South Africa, plus 17 states, two territories, and the District of Columbia in the United States and the Australian Capital Territory in Australia. In the last few weeks alone, three US states (New York, Virginia and New Mexico) have legalised cannabis for recreational purposes, all via the legislature and not via public ballots, with the intention to introduce commercial markets in the coming years. Meanwhile, the global medicinal cannabis market size was valued at GBP5.64(USD7.8bn) in 2020, and there are now 20 countries in Europe that have approved the use of medical cannabis. The IMARC Group (a leading management strategy group) estimate medicinal cannabis market growth by as much as 15.3% from 2021 to 2026.LEGAL STATUS IN JERSEY
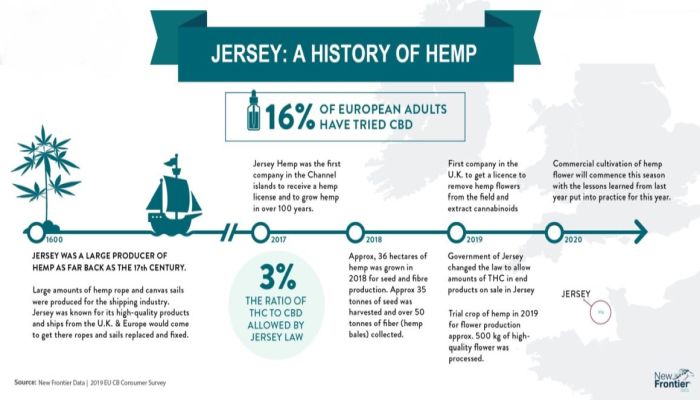
Under Jersey law, cannabinol derivatives and cannabinol, except where contained in cannabis or cannabis resin are both categorised as Class A controlled drugs under Part 1 of Schedule 2 the Misuse of Drugs (Jersey) Law 1978 (MODL), and both cannabis and cannabis resin are categorised as Class B controlled drugs under Part 2 of Schedule 2 of MODL. All these substances are further controlled under the provisions of the Misuse of Drugs (Designation) (Jersey) Order 1989 (1989 Order) and as Schedule 1 substances under the provisions of the Misuse of Drugs (General Provisions) (Jersey) Order 2009 (2009 Order). Substances placed under the control of the 1989 Order, the highest level of control, are substances for which there is generally accepted to be no therapeutic use and for which it is in the public interest that any use should only be for research or other special purposes. Any such limited activity in relation to substances controlled by the 1989 Order must be under the authority of a licence issued by the Minister for Health and Social Services. Any activity in relation to the Schedule 1 substances under the provisions of 2009 Order must again only be under the authority of a licence issued by the Health Minister, but such licenses are not necessarily restricted to research or other special purposes unless the substance is also controlled under the 1989 Order. Limited exemptions from these levels of control were introduced following amendments to the 1989 Order under the Misuse of Drugs (Miscellaneous Amendments) (No. 7) (Jersey) Order 2018, in order to enable doctors to prescribe cannabis-based products for medicinal use on 1st January 2019. Since then interest in the use of cannabis-based products for medicinal use has increased with emerging evidence of therapeutic benefits in some conditions. The Misuse of Drugs Advisory Council (MDAC) was established in accordance with Article 2 of MODL and has a duty to advise the Minister on measures, which in its opinion, should be taken to prevent the misuse of drugs and/or to deal with the social problems connected with drug abuse. Following advice from the MDAC, that keeping cannabis and related substances under the control of the 1989 Order was no longer consistent with the increasing evidence for use for medical purposes, further amendments were made to the 1989 Order under the Misuse of Drugs (Miscellaneous Amendments) (No. 8) (Jersey) Order 2019 (2019 Order). The MDAC further concluded that these substances should remain Schedule 1 substances under the provisions of 2009 Order such that any activity in relation to these substances must be authorised by licence issued by the Health Minister. CANNABINOIDS Cannabis and cannabis resin are plant products containing a wide range of chemicals generally termed cannabinoids. The most significant of these in terms of psychoactive effect is Δ9–tetrahydrocannabinol (THC). Recently there has been increased popular interest in some of the other major cannabinoids, particularly cannabidiol (CBD). A variety of health claims have been ascribed to the consumption of this compound, which is generally accepted not to have any significant psychoactive effects. In November 2020, the European Court of Justice published a judgement stating that CBD extracted from the cannabis plant should not be considered a drug under the 1961 United Nations Single Convention on Narcotic Drugs (UN Narcotic Convention). There are now numerous CBD products being marketed and sold as dietary supplements. These products are designed for the administration of CBD but almost always contain trace amounts of THC. It is virtually impossible to remove all trace amounts of THC which makes many of these products subject to control under misuse of drugs legislation unless they qualify as an exempt product as defined under 2009 Order. The MDAC concluded that it would be reasonable and proportionate to permit small amounts of THC and other cannabinoids to be present in CBD products sold as food supplements. The 2019 Order amended the 2009 Order to permit the preparation of cannabidiol which (i) has its ingredients clearly labelled, (ii) contains not more than 3% cannabinol and/or cannabinol derivatives relative to its cannabidiol content, by weight, (iii) does not contain any other controlled drug, (iv) does not contain any plant material visible to the naked eye, and (v) has the matter of the combined weight attested by an official certificate of analysis, or by the Official Analyst appointed under Article 2 of the Food Safety (Jersey) Law 1966.
Any CBD preparation meeting the above requirements should be exempted from controls on importation, possession, supply, administration. However, exportation should still be unlawful except when in accordance with a licence issued under the provisions of 2009 Order.
INVESTMENT BY JERSEY VEHICLES INTO CANNABIS AND OTHER CANNABIS-RELATED ASSETS OVERSEAS AND THE PROVISION OF RELATED FINANCIAL SERVICES
It has been recognised that the Proceeds of Crime (Jersey) Law 1999 (POCL) has the potential to create dual criminality for persons who receive income in Jersey from activities which occur legally abroad but which are unlawful in Jersey.
Jersey investors receiving income or dividends from investments in overseas medicinal cannabis companies will also need to ensure that they do not fall foul of POCL.
There are a number of offences under POCL, including:
The MDAC concluded that it would be reasonable and proportionate to permit small amounts of THC and other cannabinoids to be present in CBD products sold as food supplements. The 2019 Order amended the 2009 Order to permit the preparation of cannabidiol which (i) has its ingredients clearly labelled, (ii) contains not more than 3% cannabinol and/or cannabinol derivatives relative to its cannabidiol content, by weight, (iii) does not contain any other controlled drug, (iv) does not contain any plant material visible to the naked eye, and (v) has the matter of the combined weight attested by an official certificate of analysis, or by the Official Analyst appointed under Article 2 of the Food Safety (Jersey) Law 1966.
Any CBD preparation meeting the above requirements should be exempted from controls on importation, possession, supply, administration. However, exportation should still be unlawful except when in accordance with a licence issued under the provisions of 2009 Order.
INVESTMENT BY JERSEY VEHICLES INTO CANNABIS AND OTHER CANNABIS-RELATED ASSETS OVERSEAS AND THE PROVISION OF RELATED FINANCIAL SERVICES
It has been recognised that the Proceeds of Crime (Jersey) Law 1999 (POCL) has the potential to create dual criminality for persons who receive income in Jersey from activities which occur legally abroad but which are unlawful in Jersey.
Jersey investors receiving income or dividends from investments in overseas medicinal cannabis companies will also need to ensure that they do not fall foul of POCL.
There are a number of offences under POCL, including:
- concealing, disguising, removal, converting or transferring criminal property;
- entering into or becoming concerned in an arrangement to launder; and
- using, acquiring, possessing or controlling criminal property.

