 No matter what you build or which equipment you purchase, your business cannot be successful without hiring key staff positions and employing knowledgeable personnel. As discussed in the previous installment, almost every step in the Good Manufacturing Practices (GMP) process requires the input and approvals of competent, qualified personnel who can deliver. As you grow, your company will layer in multiple levels and supporting personnel. It is important to consider your structure ahead of time as you build up the areas of function and zero in on the recruitment process.
No matter what you build or which equipment you purchase, your business cannot be successful without hiring key staff positions and employing knowledgeable personnel. As discussed in the previous installment, almost every step in the Good Manufacturing Practices (GMP) process requires the input and approvals of competent, qualified personnel who can deliver. As you grow, your company will layer in multiple levels and supporting personnel. It is important to consider your structure ahead of time as you build up the areas of function and zero in on the recruitment process.
Corporate Structure
When your company is small, the personnel structure organization needs to be simple, yet efficient. One experienced general manager who works with a qualified person can easily manage the various functions that drive a GMP-compliant manufacturing facility. A very flat structure with few levels of hierarchy creates an environment that allows for quick decision-making and steady advancement. The most common starting structures can be seen in the chart below. As time goes on and your company grows, layers begin to form under these starting blocks, spreading managers throughout the different sectors to monitor the various processes and procedures. The two main groups employees pertinent to the GMP process fall under are Quality Assurance (QA) and Quality Control (QC), or Production.
As time goes on and your company grows, layers begin to form under these starting blocks, spreading managers throughout the different sectors to monitor the various processes and procedures. The two main groups employees pertinent to the GMP process fall under are Quality Assurance (QA) and Quality Control (QC), or Production.
Quality Assurance
The QA Team is tasked with ensuring all areas follow all the necessary procedures and standard operating practices (SOPs) and check each step of the manufacturing process. Ensuring the integrity of the process as well as the quality of the products is what regulators desire to see in order to maintain a high degree of confidence. In larger companies, the Quality Team/Unit is usually headed by a Qualified Person (QP) whose job is to hire Quality Assurance Manager, Laboratory Control Manager, and Head of Regulatory Affairs.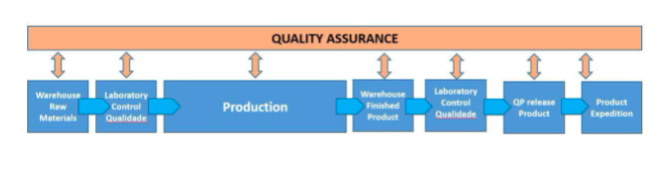 The manufacturing process begins with reception and storage of raw materials in the warehouse. Each delivery is then sampled and tested by the Laboratory of Control. The Lab Technician will analyze and approve or reject the raw materials which are received. When the materials are approved, the production teams then develop them into finished products. Meanwhile, the QA team will monitor the process and conform both in-process and results at each step. Laboratory Control takes samples to analyze and either approve or refuse. After the finished product is verified by the QA team, the product is then stored in the warehouse. Finally, the QP releases the product to the market and logistics will send to the clients.
Quality Assurance is independent of the operational execution of manufacturing. The QA can do their work without bias to the production stresses to make sure the correct process is always followed and the results are verified. The guiding mission of Quality Assurance is to verify and assure each step of finished product manufacturing is completed and recorded correctly, according to GMP, SOPs, and manufacturing instructions.
The manufacturing process begins with reception and storage of raw materials in the warehouse. Each delivery is then sampled and tested by the Laboratory of Control. The Lab Technician will analyze and approve or reject the raw materials which are received. When the materials are approved, the production teams then develop them into finished products. Meanwhile, the QA team will monitor the process and conform both in-process and results at each step. Laboratory Control takes samples to analyze and either approve or refuse. After the finished product is verified by the QA team, the product is then stored in the warehouse. Finally, the QP releases the product to the market and logistics will send to the clients.
Quality Assurance is independent of the operational execution of manufacturing. The QA can do their work without bias to the production stresses to make sure the correct process is always followed and the results are verified. The guiding mission of Quality Assurance is to verify and assure each step of finished product manufacturing is completed and recorded correctly, according to GMP, SOPs, and manufacturing instructions.
